One more Dr!Deandra passed her viva in November and we are very proud of her achievements (hence the many medals)! She worked between the Stramer and Shaw labs on a project dissecting the cellular and mechanical changes that drive keloid scarring. We wish her the best of luck for her new career in industry!
|
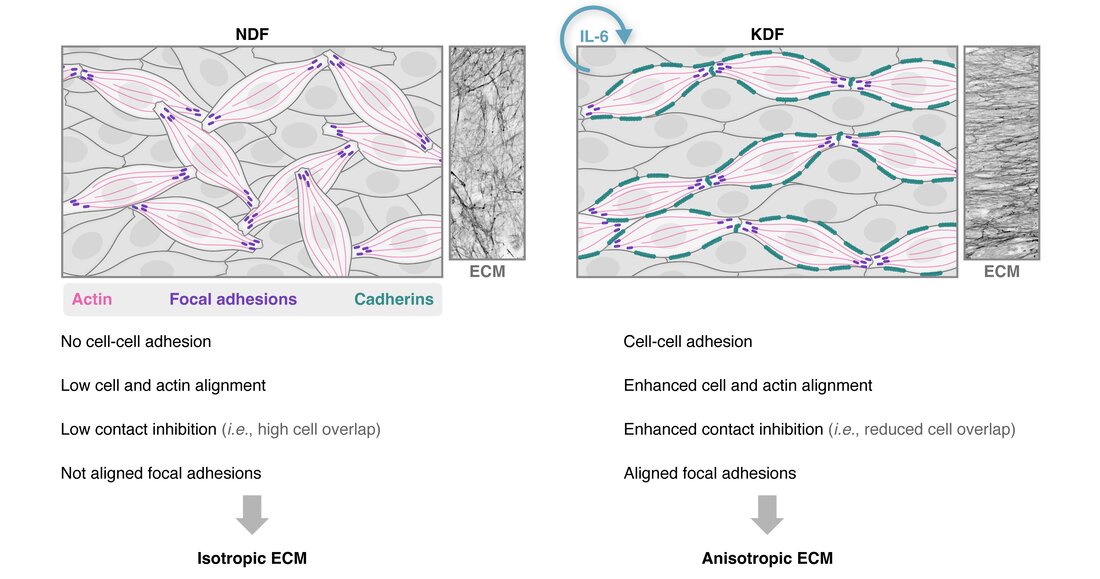
New paper out in Matrix BiologyOur new work on fibroblast and ECM anisotropy is now available open access! In this collaboration with the labs of Dr Tanya Shaw (KCL) and Dr Angelika Manhart (UCL, University of Vienna), we discuss how scar fibroblasts generate an aligned supracellular actin network, leading to ECM anisotropy. We highlight a role for autocrine interleukin-6 (IL-6) and activation of cell-cell adhesions in inducing cell and ECM alignment, using an interdisciplinary approach combining experimental work, quantitative image analysis and mathematical modelling. Here is a nice schematic made by our in-house scientific illustrator, María Díaz.
|
Stramer Lab at London PrideOn July 1st, we marched in the London Pride as part of the King's College London LGBTQ+ Staff Network contingent. We felt the love and support of our city, we are proud!
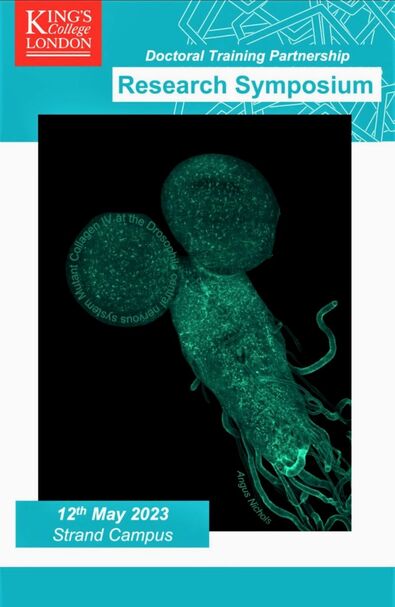
One of our images selected as advert for a meeting!An image acquired by Angus of the nervous system of the Drosophila larva was selected as cover for a meeting at King's College London. Congratulations!
|
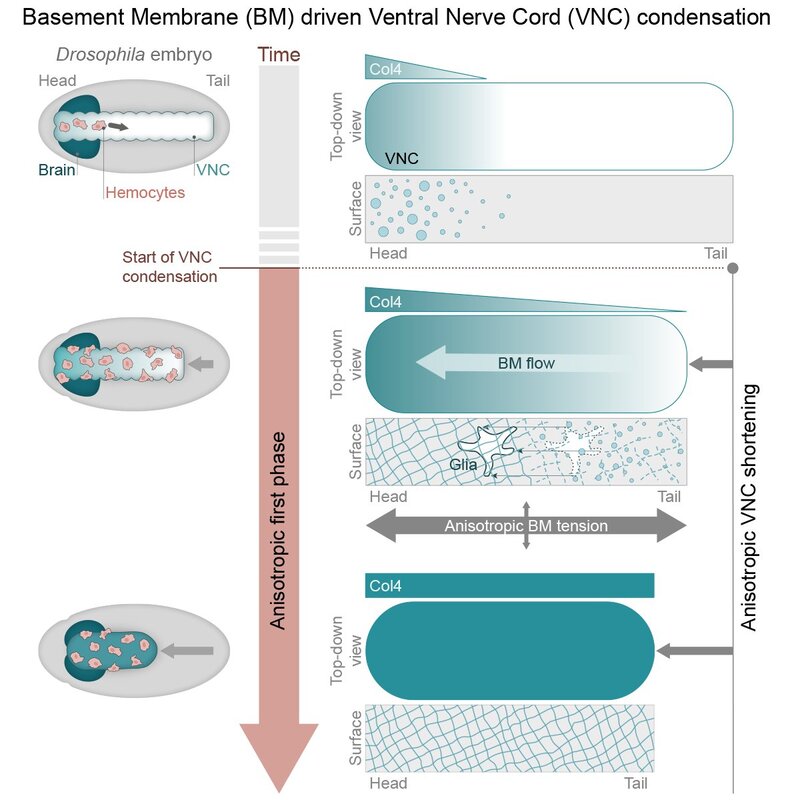
New paper on Drosophila morphogenesisA truly collaborative effort led by Eduardo resulted in the publication of our new work on Developmental Cell, where live imaging, genetic tools, image analysis and modelling were used to learn more about Drosophila morphogenesis. In this publication, we demonstrate that the initiation of Ventral Nerve Cord (VNC) condensation is triggered by sudden basement membrane assembly around the tissue, which increases the surface tension independently of cellular contractile activity. This work suggests that intrinsic stresses in an assembling basement membrane network can actively contribute forces that drive tissue morphogenesis. Have a look at the beautiful images and movies, some of which were acquired during a very fruitful visit of our lab to the Advanced Imaging Center in Janelia.
|
Our latest image analysis paper
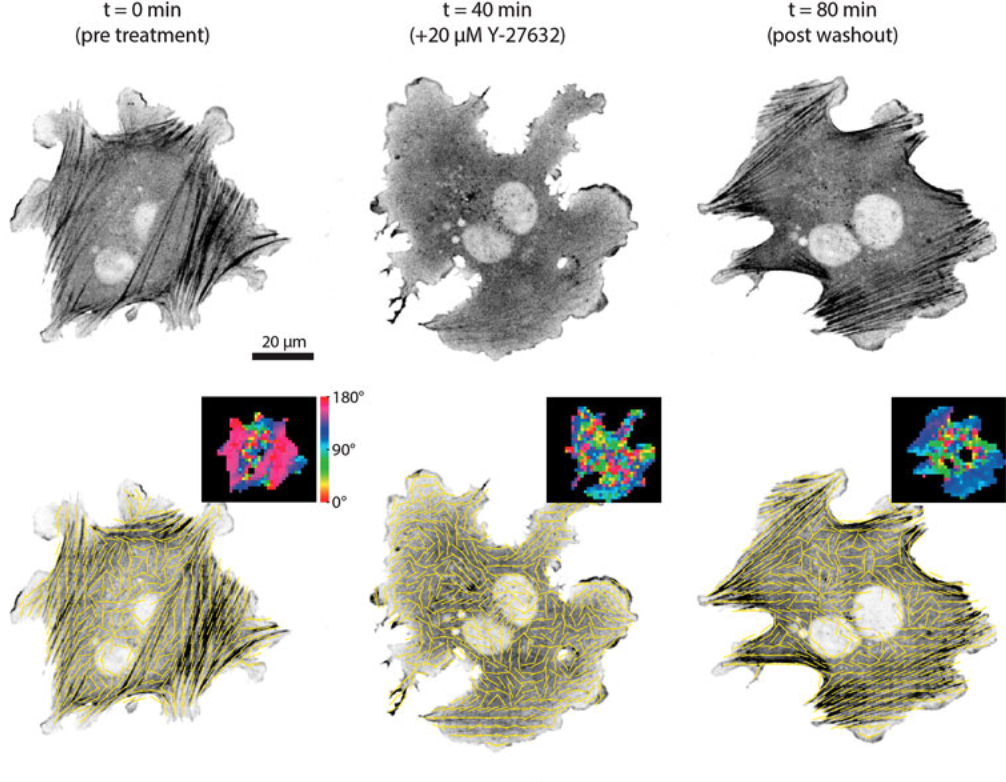
New image analysis paper out in collaboration with the Oakes Lab at Loyola University Chicago! If you are interested in quantifying alignment in bioimages, have a read: our new work comes with an open-source workflow called AFT for you to try and test on your data. You can find it in the special issue "Methods and Tools for Bioimage Analysis" of Frontiers in Computer Science - Computer vision, together with other very interesting manuscripts.
|
Forms of the Cinematic, a bit of Science History
Our group leader Brian Stramer contributed to this anthology curated by Mark E. Breeze, with a piece on microcinematography and biomedical science. Well worth a read to discover more about the history of making films through the lens of a microscope, and the big discoveries that watching biological entities move have unlocked.
On the picture, a copy of Forms of the Cinematic next to the original tools used by the pioneering cell biologist, Michael Abercrombie, one of the first scientists to use film as a quantitative tool. To know more, visit our “History of science” section here! |
New publication in STAR protocols!
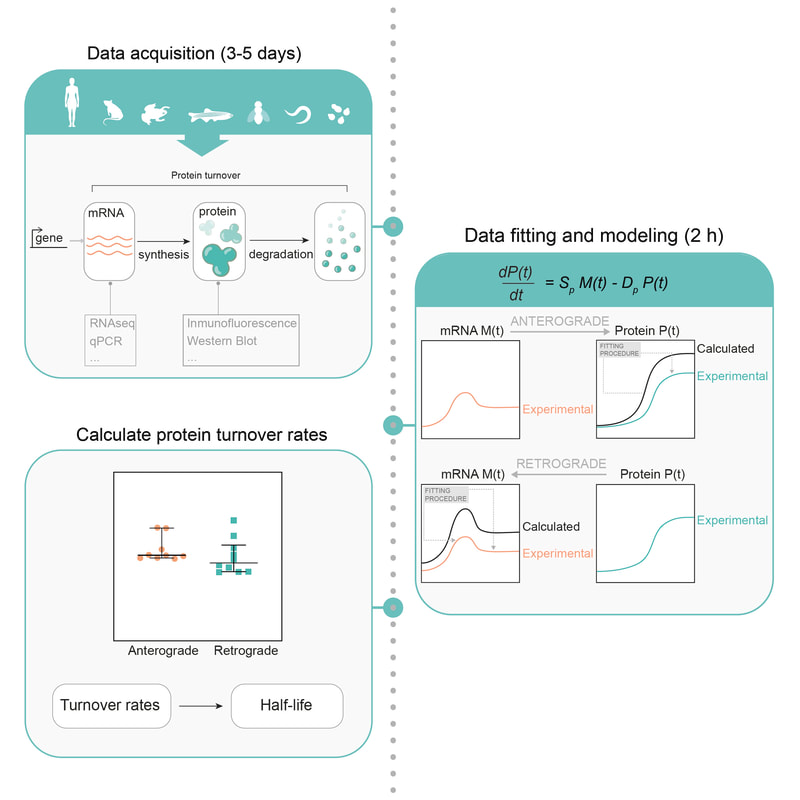
Have you read our recent publication on protein turnover (here!) and thought you would be curious to test the modelling on your data? We can help! We recently published a protocol paper in STAR Protocols (here!) to walk you through the analysis step by step. Have a look and get in touch should you have any questions!
|
|
|
Besaid's talk is out!
On December 8th 2020, our very own postdoc Besaid presented at the Cell Migration seminar series
(https://www.cellmigrationseminars.com/). You can catch up on his recorded talk on their YouTube channel by clicking in the link on the left (min 54) to hear him talking about the role of Moesin in integrating the cortical and lamellar actin networks during Drosophila macrophage migration! |
Our cover in Developmental Cell!We made it! Our cover image was selected for the latest issue of Developmental Cell, have a peep here: https://www.cell.com/developmental-cell/issue?pii=S1534-5807(19)X0014-3#
"A crocheted garment represents the extracellular matrix (ECM), which is a complex polymer network thought to form a stable cellular scaffold. The magenta patch represents the newly identified flux in ECM components." The cover image was designed by our scientific illustrator-in-residence, have a look at her portfolio here if you are interested in her work, we promise you won't be disappointed! https://www.behance.net/MariaDiaz_delaLoza |
Our last publication in Developmental Cell
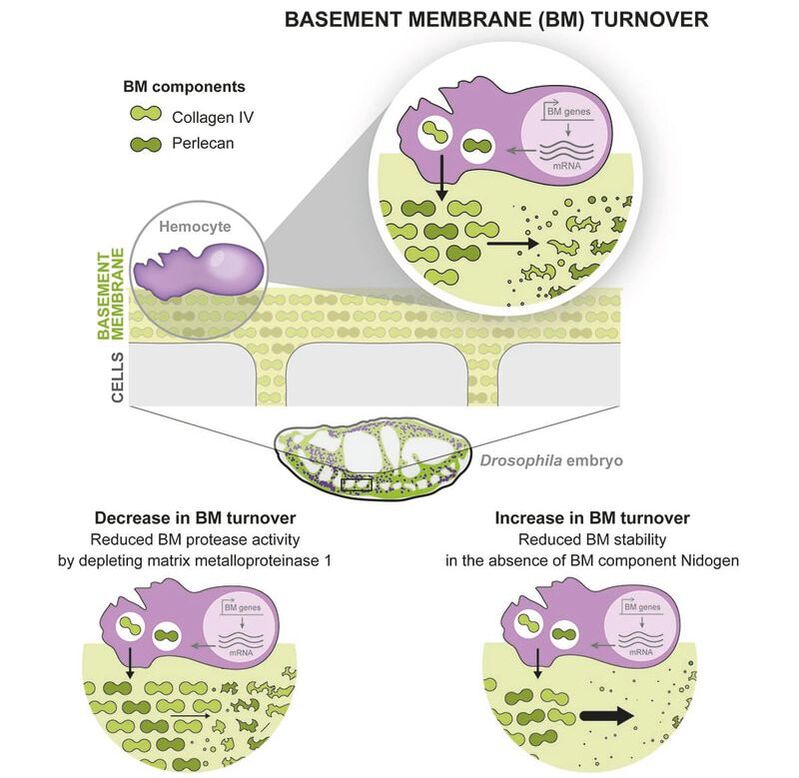
You may think the extracellular matrix is always long-lived. Check out our new paper http://zpr.io/HnJFF showing rapid turnover of ECM in embryos, a team effort combining modelling and experiment. Yutaka Matsubayashi, Besaiz Jose Sánchez-Sánchez et al. tell us about the rapid turnover of embryonic extracellular matrix during tissue morphogenesis. We imaged the de novo deposition of basement membrane in Drosophila embryos from initiation to homeostasis and mathematically modelled protein dynamics to quantify synthesis and degradation rates. The model predicted a rapid turnover of basement membrane proteins with half-lives of ~7-10 h. This finding was confirmed by in vivo pulse-chase and photoconversion experiments, thanks to a novel Collagen-Scarlet probe and a photoswitchable Collagen-EOS. Inhibiting proteolysis or ECM interactions alters the basal turnover rate, which in turn affects the normal embryonic development of the central nervous system. This rapid ECM turnover contrasts with studies in adult animals suggesting half-lives on the order of months to years. We hypothesise that a liable ECM network is likely necessary to maintain plasticity for growth and morphogenesis.
|
|
|
Developmental Dance
What if we told you that Drosophila macrophages had a sense of rhythm? And that they like some Latin groove?
If you don't believe us, look at how this amazing model for studying cell migration perform on the (embryonic) dance floor! |
Socialising (before the distancing)Here is a picture of our cheerful faces just before one of our seasonal lab socials! It was December 2019 and little did we know that --socialising (without distancing) would be put on hold for a while. We went out for Spanish food in Farringdon for Christmas (and newly published paper!) celebrations. Now we are all scattered around London working from home and we look forward to when another dinner together will be allowed. Rumour has it it’s going to be Mexican food next time…
|
Persistent and polarized global actin flow is essential for directionality during cell migration
|
Our last publication in Nature of Cell Biology
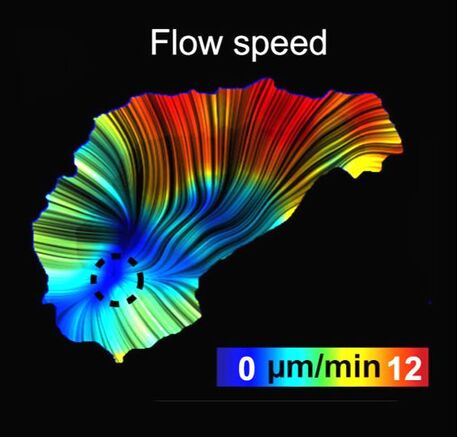
In our last paper, "Persistent and polarized global actin flow is essential for directionality during cell migration", we study the cues that determine the direction of cell migrating cells. We exploit the embryonic migration of Drosophila macrophages to bridge the different temporal scales of the behaviours controlling motility. Our results show that cell migration directionality does not correlate with the dynamics of the leading edge but with the flow of the actin network behind the leading edge. Quantification of actin flow structure during migration reveals a stable organisation and asymmetry in the cell-wide flow field that strongly correlates with cell directionality. This organisation is regulated by a gradient of actin network compression and destruction, which is controlled by myosin contraction and cofilin-mediated disassembly. It is this stable actin-flow polarity, which integrates rapid fluctuations of the leading edge, that controls inherent cellular persistence.
|
|
|
The Randall Centre for Cell & Molecular BiophysicsThe Randall Centre for Cell & Molecular Biophysics is a vibrant research environment, hosting labs working in burgeoning fields in biomedicine. Research in our Centre addresses fundamental biological questions at the interface between the physical and biomedical sciences. We develop and apply state-of-the-art biophysical techniques and address molecular and cellular processes in biology and medicine. We study these biological processes at the molecular, cellular and tissue scales.
|